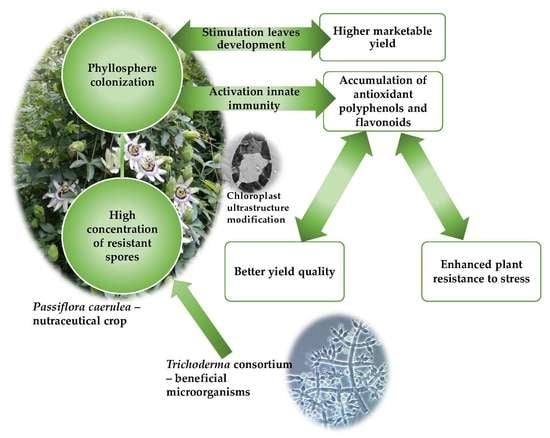
Effects of Foliar Treatment with a Trichoderma Plant Biostimulant Consortium on Passiflora caerulea L. Yield and Quality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biological Material
2.2. Field Experiment
2.3. Determination of Morphological and Physiological Characteristics of P. caerulea Plants
2.4. Ultrastructural and Morphological Analyses
2.5. Determination of Chloroplast Number and Their Surface in the Spongy Parenchyma
2.6. Determination of Total Polyphenols and Flavonoids
2.7. Antioxidant Activity Assay
2.7.1. DPPH Scavenging Activity Assay
2.7.2. Antioxidant Capacity (TEAC) Assay
2.8. Cell Culture Biocompatibility
2.9. Statistical Analysis
3. Results
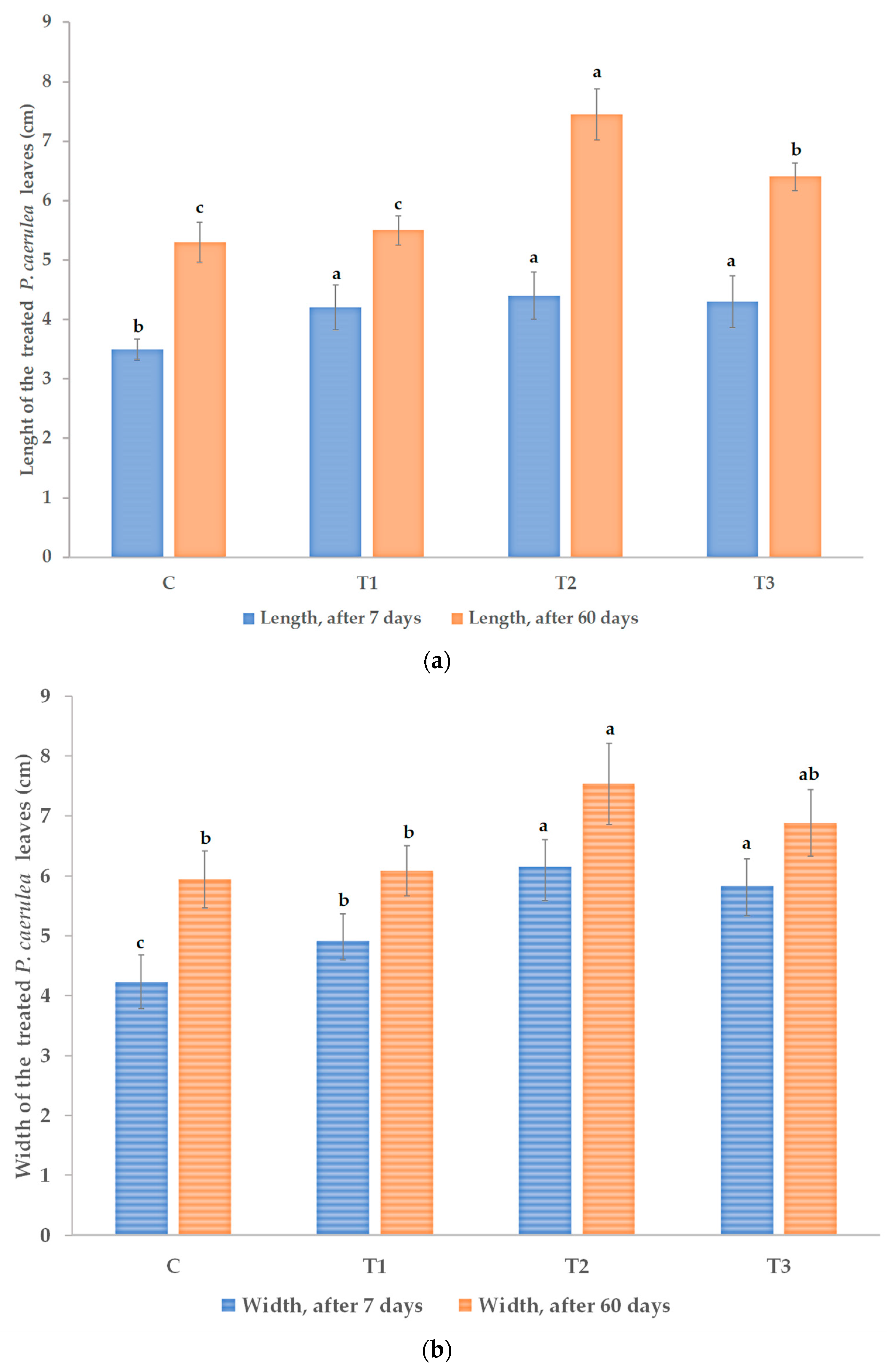
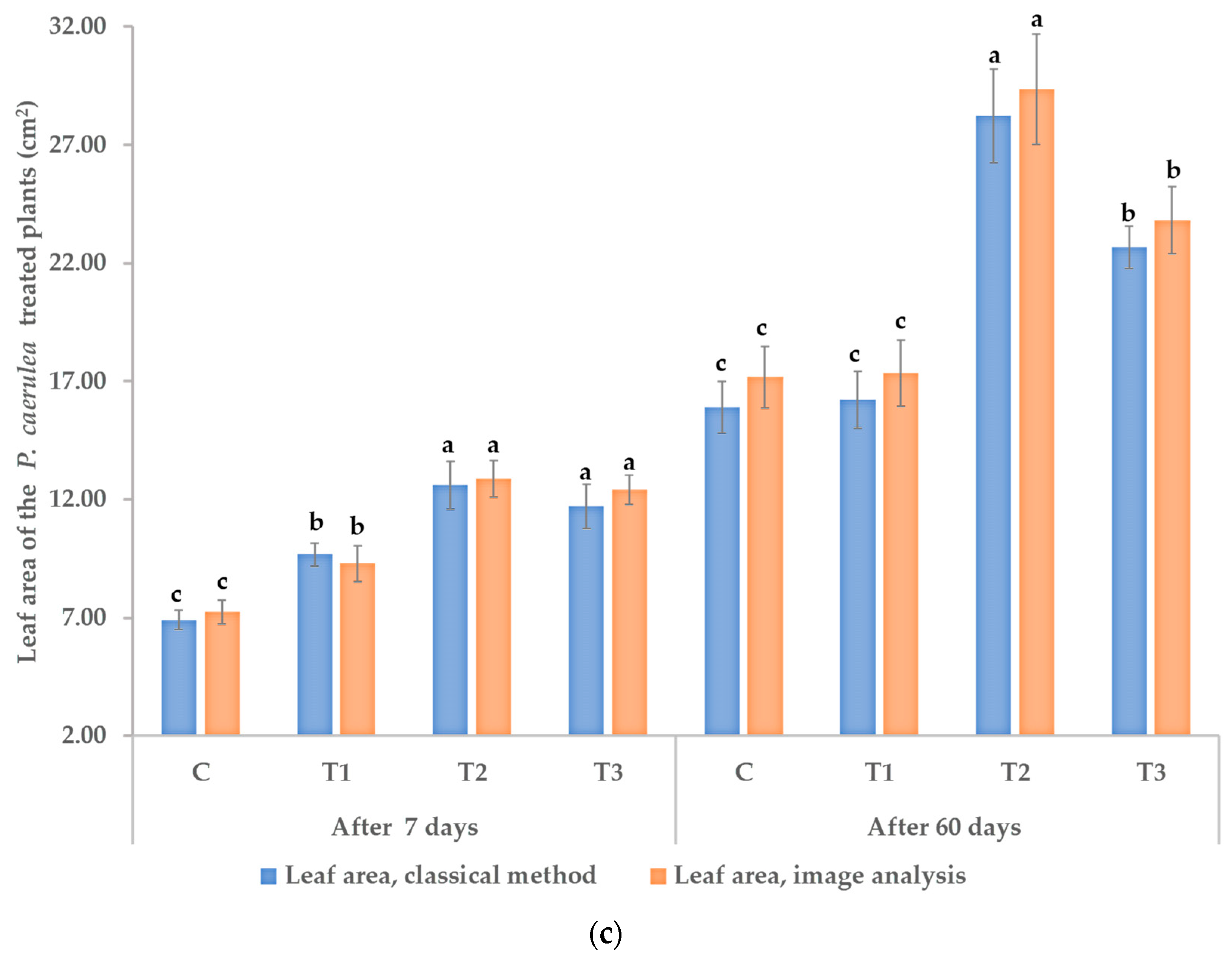
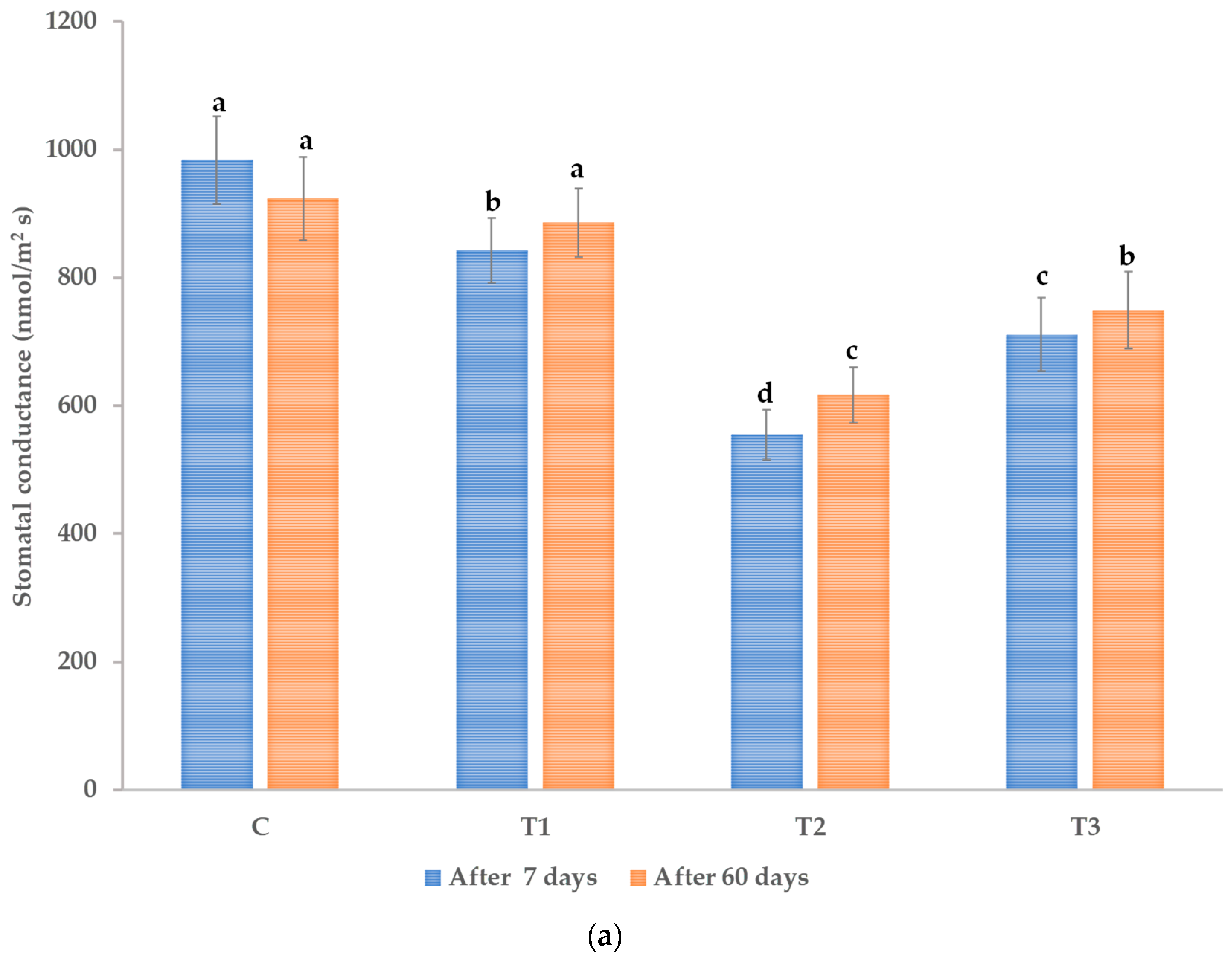
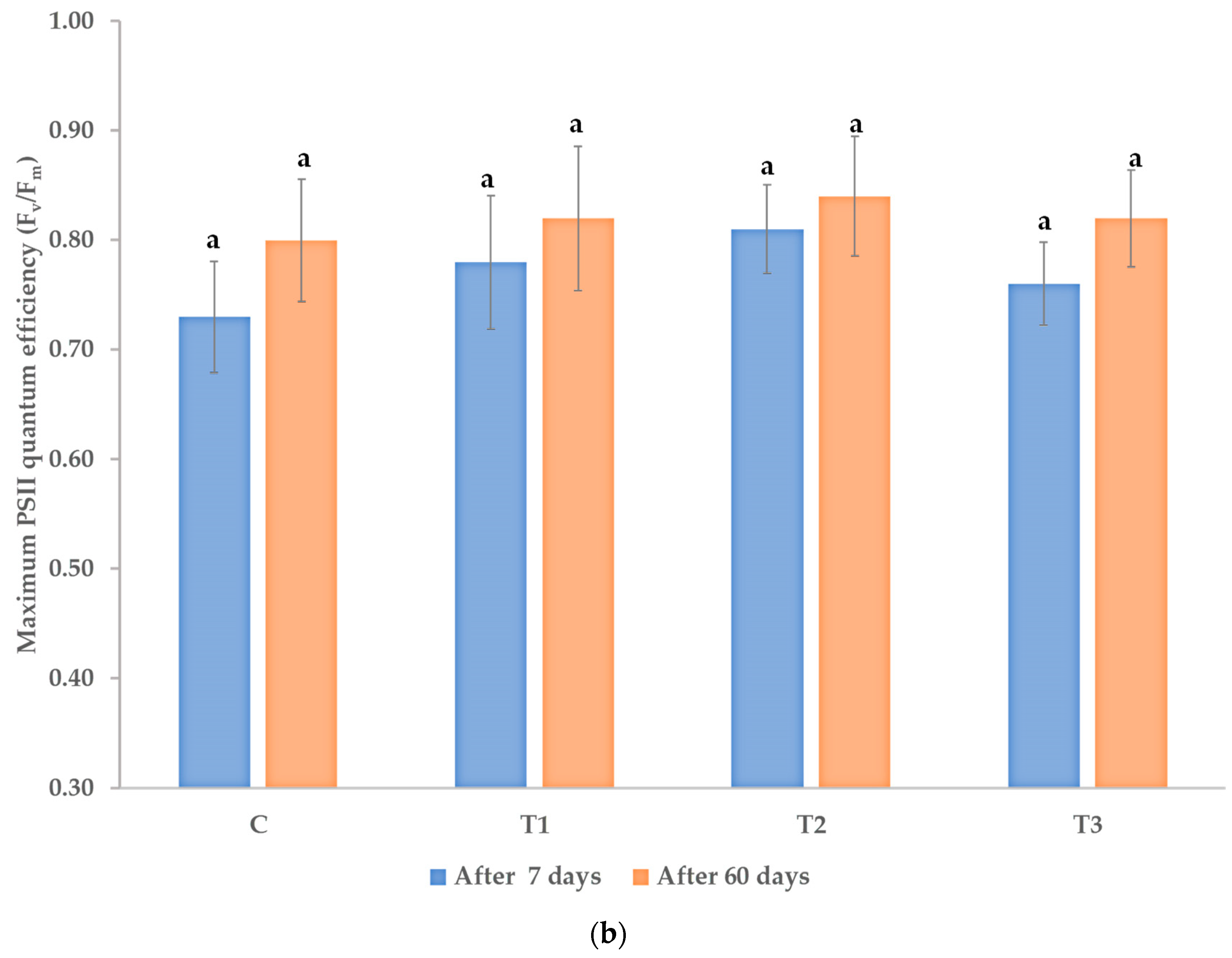
3.1. Effects on Morphological and Physiological Characteristics
3.2. Effects on Leaf Ultrastructure
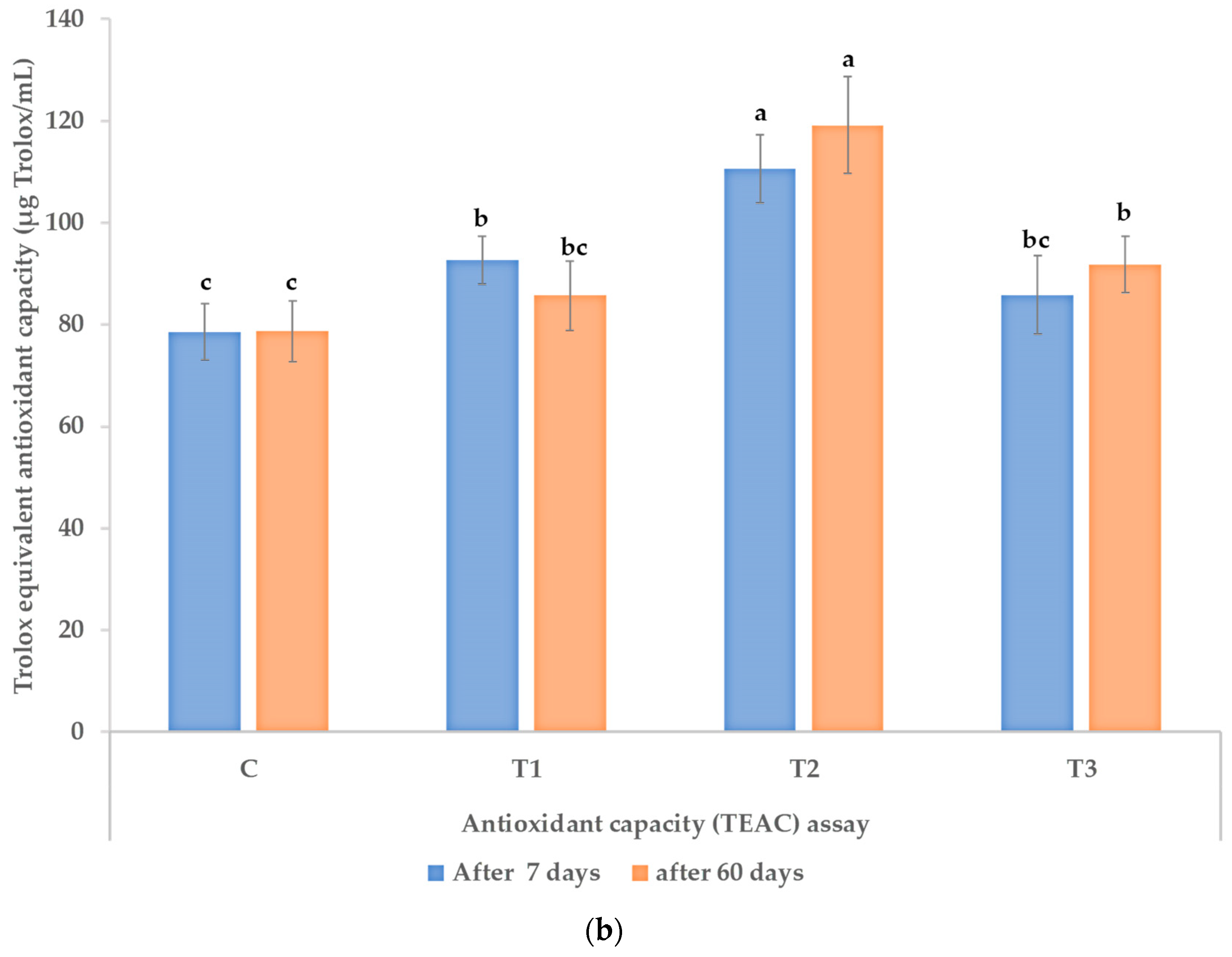
3.3. Polyphenols, Flavonoids, and Antioxidant Activity in Leaves of the Treated Plants
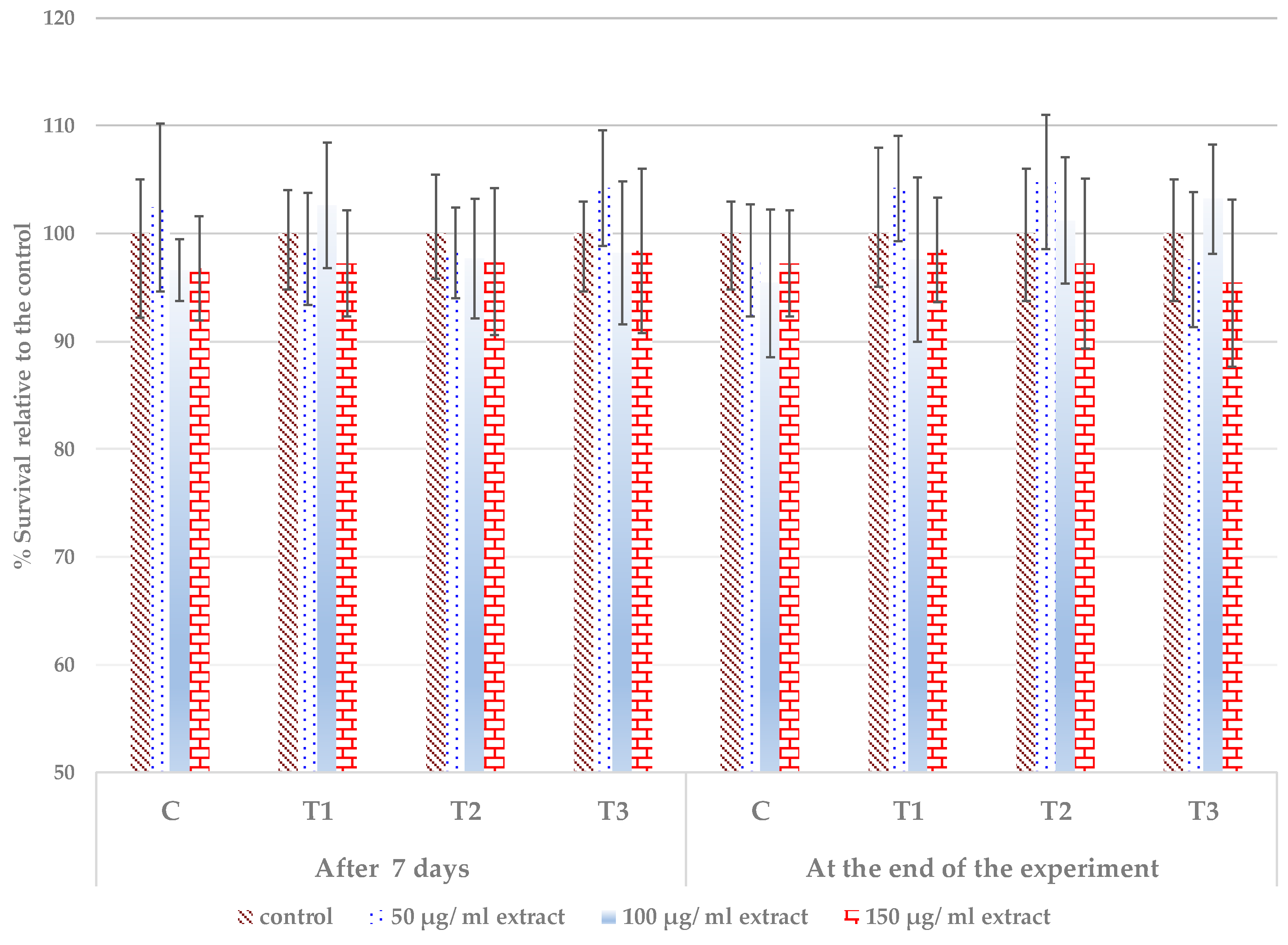
3.4. Cytocompatibility of the Extracts from the Treated Leaves
3.5. Effects on Yield and Yield Quality
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Woo, S.L.; Ruocco, M.; Vinale, F.; Nigro, M.; Marra, R.; Lombardi, N.; Pascale, A.; Lanzuise, S.; Manganiello, G.; Lorito, M. Trichoderma-based products and their widespread use in agriculture. Open Mycol. J. 2014, 8, 71–126. [Google Scholar] [CrossRef] [Green Version]
- Xue, A.G.; Guo, W.; Chen, Y.H.; Siddiqui, I.; Marchand, G.; Liu, J.H.; Ren, C.Z. Effect of seed treatment with novel strains of Trichoderma spp. on establishment and yield of spring wheat. Crop Prot. 2017, 96, 97–102. [Google Scholar] [CrossRef]
- Fiorentino, N.; Ventorino, V.; Woo, S.L.; Pepe, O.; De Rosa, A.; Gioia, L.; Romano, I.; Lombardi, N.; Napolitano, M.; Colla, G.; et al. Trichoderma-Based Biostimulants Modulate Rhizosphere Microbial Populations and Improve N Uptake Efficiency, Yield, and Nutritional Quality of Leafy Vegetables. Front. Plant Sci. 2018, 9, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowalska, J. Effects of Trichoderma asperellum T1 on Botrytis cinerea pers.: Fr., growth and yield of organic strawberry. Acta Sci. Pol.-Hortorum Cultus 2011, 10, 107–114. [Google Scholar]
- Kaewchai, S.; Soytong, K.; Hyde, K.D. Mycofungicides and fungal biofertilizers. Fungal Divers. 2009, 38, 25–50. [Google Scholar]
- Vos, C.M.F.; De Cremer, K.; Cammue, B.P.A.; De Coninck, B. The toolbox of Trichoderma spp. in the biocontrol of Botrytis cinerea disease. Mol. Plant Pathol. 2015, 16, 400–412. [Google Scholar] [CrossRef] [Green Version]
- López-Bucio, J.; Pelagio-Flores, R.; Herrera-Estrella, A. Trichoderma as biostimulant: Exploiting the multilevel properties of a plant beneficial fungus. Sci. Hortic. 2015, 196, 109–123. [Google Scholar] [CrossRef]
- Chen, M.; Liu, Q.; Gao, S.-S.; Young, A.E.; Jacobsen, S.E.; Tang, Y. Genome mining and biosynthesis of a polyketide from a biofertilizer fungus that can facilitate reductive iron assimilation in plant. Proc. Natl. Acad. Sci. USA 2019, 116, 5499–5504. [Google Scholar] [CrossRef] [Green Version]
- Verma, M.; Brar, S.K.; Tyagi, R.D.; Surampalli, R.Y.; Valero, J.R. Antagonistic fungi, Trichoderma spp.: Panoply of biological control. Biochem. Eng. J. 2007, 37, 1–20. [Google Scholar] [CrossRef]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species - Opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef]
- Hermosa, R.; Viterbo, A.; Chet, I.; Monte, E. Plant-beneficial effects of Trichoderma and of its genes. Microbiology 2012, 158, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Hermosa, R.; Rubio, M.B.; Cardoza, R.E.; Nicolas, C.; Monte, E.; Gutierrez, S. The contribution of Trichoderma to balancing the costs of plant growth and defense. Int. Microbiol. 2013, 16, 69–80. [Google Scholar] [CrossRef]
- Gaderer, R.; Lamdan, N.L.; Frischmann, A.; Sulyok, M.; Krska, R.; Horwitz, B.A.; Seidl-Seiboth, V. Sm2, a paralog of the Trichoderma cerato-platanin elicitor Sm1, is also highly important for plant protection conferred by the fungal-root interaction of Trichoderma with maize. BMC Microbiol. 2015, 15, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, E.V.; Costa, M.D.; de Paula, R.G.; de Azevedo, R.R.; da Silva, F.L.; Noronha, E.F.; Ulhoa, C.J.; Monteiro, V.N.; Cardoza, R.E.; Gutierrez, S.; et al. The Cerato-Platanin protein Epl-1 from Trichoderma harzianum is involved in mycoparasitism, plant resistance induction and self cell wall protection. Sci. Rep. 2015, 5, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nawrocka, J.; Malolepsza, U. Diversity in plant systemic resistance induced by Trichoderma. Biol. Control 2013, 67, 149–156. [Google Scholar] [CrossRef]
- Singh, B.N.; Dwivedi, P.; Sarma, B.K.; Singh, G.S.; Singh, H.B. Trichoderma asperellum T42 Reprograms Tobacco for Enhanced Nitrogen Utilization Efficiency and Plant Growth When Fed with N Nutrients. Front. Plant Sci. 2018, 9, 15. [Google Scholar] [CrossRef]
- Pelagio-Flores, R.; Esparza-Reynoso, S.; Garnica-Vergara, A.; Lopez-Bucio, J.; Herrera-Estrella, A. Trichoderma-Induced Acidification Is an Early Trigger for Changes in Arabidopsis Root Growth and Determines Fungal Phytostimulation. Front. Plant Sci. 2017, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Garnica-Vergara, A.; Barrera-Ortiz, S.; Munoz-Parra, E.; Raya-Gonzalez, J.; Mendez-Bravo, A.; Macias-Rodriguez, L.; Ruiz-Herrera, L.F.; Lopez-Bucio, J. The volatile 6-pentyl-2H-pyran-2-one from Trichoderma atroviride regulates Arabidopsis thaliana root morphogenesis via auxin signaling and ETHYLENE INSENSITIVE 2 functioning. New Phytol. 2016, 209, 1496–1512. [Google Scholar] [CrossRef] [Green Version]
- Coppola, M.; Diretto, G.; Digilio, M.C.; Woo, S.L.; Giuliano, G.; Molisso, D.; Pennacchio, F.; Lorito, M.; Rao, R. Transcriptome and Metabolome Reprogramming in Tomato Plants by Trichoderma harzianum strain T22 Primes and Enhances Defense Responses Against Aphids. Front. Physiol. 2019, 10. [Google Scholar] [CrossRef]
- Mona, S.A.; Hashem, A.; Abd_Allah, E.F.; Alqarawi, A.A.; Soliman, D.W.K.; Wirth, S.; Egamberdieva, D. Increased resistance of drought by Trichoderma harzianum fungal treatment correlates with increased secondary metabolites and proline content. J. Integr. Agric. 2017, 16, 1751–1757. [Google Scholar] [CrossRef]
- Sánchez-Montesinos, B.; Diánez, F.; Moreno-Gavira, A.; Gea, F.J.; Santos, M. Plant Growth Promotion and Biocontrol of Pythium ultimum by Saline Tolerant Trichoderma Isolates under Salinity Stress. Int. J. Environ. Res. Public Health 2019, 16, 2053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghorbanpour, A.; Salimi, A.; Ghanbary, M.A.T.; Pirdashti, H.; Dehestani, A. The effect of Trichoderma harzianum in mitigating low temperature stress in tomato (Solanum lycopersicum L.) plants. Sci. Hortic. 2018, 230, 134–141. [Google Scholar] [CrossRef]
- Tripathi, P.; Singh, P.C.; Mishra, A.; Chauhan, P.S.; Dwivedi, S.; Bais, R.T.; Tripathi, R.D. Trichoderma: A potential bioremediator for environmental clean up. Clean Technol. Environ. Policy 2013, 15, 541–550. [Google Scholar] [CrossRef]
- Harman, G.E.; Doni, F.; Khadka, R.B.; Uphoff, N. Endophytic strains of Trichoderma increase plants’ photosynthetic capability. J. Appl. Microbiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Lopez, A.; Recena, R.; Aviles, M.; Delgado, A. Effect of Bacillus subtilis QST713 and Trichoderma asperellum T34 on P uptake by wheat and how it is modulated by soil properties. J. Soils Sediments 2018, 18, 727–738. [Google Scholar] [CrossRef]
- Li, R.X.; Cai, F.; Pang, G.; Shen, Q.R.; Li, R.; Chen, W. Solubilisation of Phosphate and Micronutrients by Trichoderma harzianum and Its Relationship with the Promotion of Tomato Plant Growth. PLoS ONE 2015, 10, 16. [Google Scholar] [CrossRef] [Green Version]
- de Santiago, A.; Quintero, J.M.; Aviles, M.; Delgado, A. Effect of Trichoderma asperellum strain T34 on iron, copper, manganese, and zinc uptake by wheat grown on a calcareous medium. Plant Soil 2011, 342, 97–104. [Google Scholar] [CrossRef]
- Martinez-Medina, A.; Van Wees, S.C.M.; Pieterse, C.M.J. Airborne signals from Trichoderma fungi stimulate iron uptake responses in roots resulting in priming of jasmonic acid-dependent defences in shoots of Arabidopsis thaliana and Solanum lycopersicum. Plant Cell Environ. 2017, 40, 2691–2705. [Google Scholar] [CrossRef] [Green Version]
- Akter, Z.; Neumann, G.; Romheld, V. Effects of biofertilizers on mn and zn acquisition and growth of higher plants: A rhizobox experiment. J. Plant Nutr. 2015, 38, 596–608. [Google Scholar] [CrossRef]
- Goicoechea, N.; Antolin, M.C. Increased nutritional value in food crops. Microb. Biotechnol. 2017, 10, 1004–1007. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Davis, L.C.; Verpoorte, R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol. Adv. 2005, 23, 283–333. [Google Scholar] [CrossRef] [PubMed]
- Gertsch, J. The metabolic plant feedback hypothesis: How plant secondary metabolites nonspecifically impact human health. Planta Med. 2016, 82, 920–929. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.; Singh, A.; Gupta, M.M.; Pandey, R. Cumulative role of bioinoculants on growth, antioxidant potential and artemisinin content in Artemisia annua L. under organic field conditions. World J. Microbiol. Biotechnol. 2016, 32, 10. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Singh, H.B.; Singh, D.K. Effect of Trichoderma harzianum on Mineral Component and Antioxidant Activity of Tomato Fruits. Vegetos 2013, 26, 237–244. [Google Scholar] [CrossRef]
- Pascale, A.; Vinale, F.; Manganiello, G.; Nigro, M.; Lanzuise, S.; Ruocco, M.; Marra, R.; Lombardi, N.; Woo, S.L.; Lorito, M. Trichoderma and its secondary metabolites improve yield and quality of grapes. Crop Prot. 2017, 92, 176–181. [Google Scholar] [CrossRef] [Green Version]
- Rouphael, Y.; Colla, G.; Graziani, G.; Ritieni, A.; Cardarelli, M.; De Pascale, S. Phenolic composition, antioxidant activity and mineral profile in two seed-propagated artichoke cultivars as affected by microbial inoculants and planting time. Food Chem. 2017, 234, 10–19. [Google Scholar] [CrossRef]
- Ortega-Garcia, J.G.; Montes-Belmont, R.; Rodriguez-Monroy, M.; Ramirez-Trujillo, J.A.; Suarez-Rodriguez, R.; Sepulveda-Jimenez, G. Effect of Trichoderma asperellum applications and mineral fertilization on growth promotion and the content of phenolic compounds and flavonoids in onions. Sci. Hortic. 2015, 195, 8–16. [Google Scholar] [CrossRef]
- Nawrocka, J.; Szczech, M.; Malolepsza, U. Trichoderma atroviride Enhances Phenolic Synthesis and Cucumber Protection against Rhizoctonia solani. Plant Prot. Sci. 2018, 54, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Zeraik, M.L.; Pereira, C.A.M.; Zuin, V.G.; Yariwake, J.H. Passion fruit: A functional food? Rev. Bras. Farmacogn.-Braz. J. Pharmacogn. 2010, 20, 459–471. [Google Scholar] [CrossRef] [Green Version]
- Miyasaka, L.S.; Atallah, Á.N.; Soares, B. Passiflora for anxiety disorder. Cochrane Database Syst. Rev. 2007. [Google Scholar] [CrossRef]
- Deng, J.; Zhou, Y.J.; Bai, M.M.; Li, H.W.; Li, L. Anxiolytic and sedative activities of Passiflora edulis f. flavicarpa. J. Ethnopharmacol. 2010, 128, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, F.A.; Medina, G.M. Effect of a medicinal plant (Passiflora incarnata) on sleep. Sleep Sci. 2017, 10, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Anzoise, M.L.; Marrassini, C.; Bach, H.; Gorzalczany, S. Beneficial properties of Passiflora caerulea on experimental colitis. J. Ethnopharmacol. 2016, 194, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Miroddi, M.; Calapai, G.; Navarra, M.; Minciullo, P.L.; Gangemi, S. Passiflora incarnata L.: Ethnopharmacology, clinical application, safety and evaluation of clinical trials. J. Ethnopharmacol. 2013, 150, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Sudasinghe, H.P.; Peiris, D.C. Hypoglycemic and hypolipidemic activity of aqueous leaf extract of Passiflora suberosa L. PeerJ 2018, 6, e4389. [Google Scholar] [CrossRef] [PubMed]
- Konta, E.M.; Almeida, M.R.; do Amaral, C.L.; Darin, J.D.C.; de Rosso, V.V.; Mercadante, A.Z.; Antunes, L.M.G.; Bianchi, M.L.P. Evaluation of the Antihypertensive Properties of Yellow Passion Fruit Pulp (Passiflora edulis Sims f. flavicarpa Deg.) in Spontaneously Hypertensive Rats. Phytother. Res. 2014, 28, 28–32. [Google Scholar] [CrossRef]
- Schunck, R.V.A.; Macedo, I.C.; Laste, G.; de Souza, A.; Valle, M.T.C.; Salomon, J.L.O.; Nunes, E.A.; Campos, A.C.W.; Gnoatto, S.C.B.; Bergold, A.M.; et al. Standardized Passiflora incarnata L. Extract Reverts the Analgesia Induced by Alcohol Withdrawal in Rats. Phytother. Res. 2017, 31, 1199–1208. [Google Scholar] [CrossRef]
- Breivogel, C.; Jamerson, B. Passion flower extract antagonizes the expression of nicotine locomotor sensitization in rats. Pharm. Biol. 2012, 50, 1310–1316. [Google Scholar] [CrossRef] [Green Version]
- Gadioli, I.L.; da Cunha, M.D.B.; de Carvalho, M.V.O.; Costa, A.M.; Pineli, L.D.D. A systematic review on phenolic compounds in Passiflora plants: Exploring biodiversity for food, nutrition, and popular medicine. Crit. Rev. Food Sci. Nutr. 2018, 58, 785–807. [Google Scholar] [CrossRef]
- Argentieri, M.P.; Levi, M.; Guzzo, F.; Avato, P. Phytochemical analysis of Passiflora loefgrenii Vitta, a rich source of luteolin-derived flavonoids with antioxidant properties. J. Pharm. Pharmacol. 2015, 67, 1603–1612. [Google Scholar] [CrossRef]
- da Silva, J.K.; Cazarin, C.B.B.; Colomeu, T.C.; Batista, Â.G.; Meletti, L.M.M.; Paschoal, J.A.R.; Bogusz Júnior, S.; Furlan, M.F.; Reyes, F.G.R.; Augusto, F.; et al. Antioxidant activity of aqueous extract of passion fruit (Passiflora edulis) leaves: In vitro and in vivo study. Food Res. Int. 2013, 53, 882–890. [Google Scholar] [CrossRef] [Green Version]
- Bendini, A.; Cerretani, L.; Pizzolante, L.; Toschi, T.G.; Guzzo, F.; Ceoldo, S.; Marconi, A.M.; Andreetta, F.; Levi, M. Phenol content related to antioxidant and antimicrobial activities of Passiflora spp. extracts. Eur. Food Res. Technol. 2006, 223, 102–109. [Google Scholar] [CrossRef]
- Lugato, D.; Simo, M.J.; Garcia, R.; Mansur, E.; Pacheco, G. Determination of antioxidant activity and phenolic content of extracts from in vivo plants and in vitro materials of Passiflora alata Curtis. Plant Cell Tissue Organ Cult. 2014, 118, 339–346. [Google Scholar] [CrossRef]
- Nguyen, T.Y.; To, D.C.; Tran, M.H.; Lee, J.S.; Lee, J.H.; Kim, J.A.; Woo, M.H.; Min, B.S. Anti-inflammatory Flavonoids Isolated from Passiflora foetida. Nat. Prod. Commun. 2015, 10, 929–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saravanan, S.; Arunachalam, K.; Parimelazhagan, T. Antioxidant, analgesic, anti-inflammatory and antipyretic effects of polyphenols from Passiflora subpeltata leaves - A promising species of Passiflora. Ind. Crop. Prod. 2014, 54, 272–280. [Google Scholar] [CrossRef]
- Sasikala, V.; Saravanan, S.; Parimelazhagan, T. Analgesic and anti-inflammatory activities of Passiflora foetida L. Asian Pac. J. Trop. Med. 2011, 4, 600–603. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, M.A.G.; Rehn, K.G.; Menezes, M.; Mariano, R. Antagonism of Trichoderma species on Cladosporium herbarum and their enzimatic characterization. Braz. J. Microbiol. 2001, 32, 98–104. [Google Scholar] [CrossRef] [Green Version]
- Fischer, I.H.; de Almeida, A.M.; Fileti, M.D.; Bertani, R.M.D.; de Arruda, M.C.; Bueno, C.J. Evaluation of Passifloraceas, fungicides and Trichoderma for passion fruit collar rot handling, caused by Nectria haematococca. Rev. Bras. De Frutic. 2010, 32, 709–717. [Google Scholar] [CrossRef] [Green Version]
- dos Santos, H.A.; Mello, S.C.M.; Peixoto, J.R. Association of isolates of Trichoderma spp. and indole-3-butyric acid (iba) in promoting root and growth of Passion. Biosci. J. 2010, 26, 966–972. [Google Scholar]
- Hassani, M.A.; Duran, P.; Hacquard, S. Microbial interactions within the plant holobiont. Microbiome 2018, 6, 17. [Google Scholar] [CrossRef]
- Yuan, M.; Huang, Y.; Jia, Z.; Ge, W.; Zhang, L.; Zhao, Q.; Song, S.; Huang, Y. Whole RNA-sequencing and gene expression analysis of Trichoderma harzianum Tr-92 under chlamydospore-producing condition. Genes Genom. 2019, 41, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Răut, I.; Badea-Doni, M.; Calin, M.; Oancea, F.; Vasilescu, G.; Sesan, T.E.; Jecu, L. Effect of volatile and non-volatile metabolites from Trichoderma spp. Against important phytopathogens. Rev Chim-Buchar. 2014, 65, 1285–1288. [Google Scholar]
- Oancea, F.; Mara, G.; Sesan, T.; Máthé, I.; Raut, J.; Ábrahám, B.; Lányi, S. Strain of Trichoderma harzianum and Controlled Release Composition Which Contains Said Strain. Patent RO 128889 B1, 30 June 2017. [Google Scholar]
- Răut, I.; Calin, M.; Vasilescu, G.; Doni, M.; Sesan, T.; Jecu, L. Effect of non volatile compounds of Trichoderma spp. against Fusarium graminearum, Rhizoctonia solani and Pythium ultimum. Sci. Bull. Ser. F. Biotechnol. 2014, 18, 178–181. [Google Scholar]
- Răut, I.; Sesan, T.E.; Macias, R.; Doni, M.B.; Oancea, F.; Calin, M.; Arsene, M.L.; Vasilescu, G.; Jecu, L. Study on the effectiveness of antagonistic Trichoderma spp. on the growth of some vegetables under laboratory and greenhouse conditions. Rev. Chim-Buchar. 2016, 67, 1504–1507. [Google Scholar]
- Răut, I.; Oancea, F.; Sesan, T.E.; Jecu, L.; Arsene, M.L.; Doni, M.B.; Vasilescu, G. Trichoderma asperellum Td36-Versatile strain for treatment of high residue agricultural systems and nutraceutical crops. J. Biotechnol. 2015, 208, S62. [Google Scholar] [CrossRef]
- Oancea, F.; Raut, I.; Șesan, T.E.; Cornea, P.C.; Badea-Doni, M.; Popescu, M.; Jecu, M.L. Hydro-gelified and film forming formulation of microbial plant biostimulants for crop residues treatment on conservation agriculture systems. Studia Univ. Vasile Goldis Ser. Stiintele Vietii (Life Sci. Ser.) 2016, 26, 251–260. [Google Scholar]
- ZamfiropoL-Cristea, V.; Răut, I.; Șeșan, T.E.; Trică, B.; Oancea, F. Surface response optimization of submerged biomass production for a plant biostimulant trichoderma strain. Sci. Bull. Ser. F. Biotechnol. 2017, 21, 56–65. [Google Scholar]
- Oancea, F.; Raut, I.; Şesan, T.E.; Cornea, P.C. Dry flowable formulation of biostimulants Trichoderma strains. Agric. Agric. Sci. Procedia 2016, 10, 494–502. [Google Scholar] [CrossRef] [Green Version]
- Aberkane, A.; Cuenca-Estrella, M.; Gomez-Lopez, A.; Petrikkou, E.; Mellado, E.; Monzon, A.; Rodriguez-Tudela, J. Comparative evaluation of two different methods of inoculum preparation for antifungal susceptibility testing of filamentous fungi. J. Antimicrob. Chemother. 2002, 50, 719–722. [Google Scholar] [CrossRef] [Green Version]
- Rouphael, Y.; Giordano, M.; Cardarelli, M.; Cozzolino, E.; Mori, M.; Kyriacou, M.C.; Bonini, P.; Colla, G. Plant- and Seaweed-Based Extracts Increase Yield but Differentially Modulate Nutritional Quality of Greenhouse Spinach through Biostimulant Action. Agronomy 2018, 8, 126. [Google Scholar] [CrossRef] [Green Version]
- Sârbu, A.; Paraschiv, M.A.; Oancea, F.; Şesan, T.E. Passiflora caerulea L. Treated with Trichoderma plant biostimulants consortium. Morpho-anatomical considerations. J. Plant Dev. 2018, 25, 2–14. [Google Scholar]
- Sesan, T.E.; Sârbu, A.; Smarandache, D.; Oancea, F.; Oancea, A.; Savin, S.; Toma, A.; Stefan, L.; Negru, G.; Bira, A.F. Botanical and Phytochemical Approach on Passiflora Spp.-New Nutraceutical Crop in Romania. J. Plant Dev. 2016, 23, 97–127. [Google Scholar]
- Plotze, R.d.O.; Falvo, M.; Pádua, J.G.; Bernacci, L.C.; Vieira, M.L.C.; Oliveira, G.C.X.; Bruno, O.M. Leaf shape analysis using the multiscale Minkowski fractal dimension, a new morphometric method: A study with Passiflora (Passifloraceae). Can. J. Bot. 2005, 83, 287–301. [Google Scholar] [CrossRef]
- Morgado, M.A.D.O.; Bruckner, C.H.; Rosado, L.D.S.; Assunção, W.; dos Santos, C.E.M. Estimação da área foliar por método não destrutivo, utilizando medidas lineares das folhas de espécies de Passiflora. Rev. Ceres 2015, 60, 662–667. [Google Scholar] [CrossRef] [Green Version]
- Zechmann, B. Ultrastructure of plastids serves as reliable abiotic and biotic stress marker. PLoS ONE 2019, 14, e0214811. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Craciunescu, O.; Constantin, D.; Gaspar, A.; Toma, L.; Utoiu, E.; Moldovan, L. Evaluation of antioxidant and cytoprotective activities of Arnica montana L. and Artemisia absinthium L. ethanolic extracts. Chem. Cent. J. 2012, 6, 97. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Repetto, G.; Del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Cornejo, H.A.; Macias-Rodriguez, L.; Vergara, A.G.; Lopez-Bucio, J. Trichoderma Modulates Stomatal Aperture and Leaf Transpiration Through an Abscisic Acid-Dependent Mechanism in Arabidopsis. J. Plant Growth Regul. 2015, 34, 425–432. [Google Scholar] [CrossRef]
- Fiorini, L.; Guglielminetti, L.; Mariotti, L.; Curadi, M.; Picciarelli, P.; Scartazza, A.; Sarrocco, S.; Vannacci, G. Trichoderma harzianum T6776 modulates a complex metabolic network to stimulate tomato cv. Micro-Tom growth. Plant Soil 2016, 400, 351–366. [Google Scholar] [CrossRef]
- El-Bakry, H.A.; El-Sherif, G.; Rostom, R.M. Therapeutic dose of green tea extract provokes liver damage and exacerbates paracetamol-induced hepatotoxicity in rats through oxidative stress and caspase 3-dependent apoptosis. Biomed. Pharmacother. 2017, 96, 798–811. [Google Scholar] [CrossRef] [PubMed]
- Kocyigit, A.; Selek, S. Exogenous Antioxidants are Double-edged Swords. Bezmialem Sci. 2016, 4, 70–75. [Google Scholar] [CrossRef]
- Jain, A.K.; Singh, D.; Dubey, K.; Maurya, R.; Mittal, S.; Pandey, A.K. Models and methods for in vitro toxicity. In In Vitro Toxicology; Dhawan, A., Kwon, S., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 45–65. [Google Scholar] [CrossRef]
- Krishna, G.; Gopalakrishnan, G. Alternative in vitro models for safety and toxicity evaluation of nutraceuticals. In Nutraceuticals; Gupta, R.C., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 355–385. [Google Scholar] [CrossRef]
- Uțoiu, E.; Matei, F.; Toma, A.; Diguță, C.F.; Ștefan, L.M.; Mănoiu, S.; Vrăjmașu, V.V.; Moraru, I.; Oancea, A.; Israel-Roming, F.; et al. Bee Collected Pollen with Enhanced Health Benefits, Produced by Fermentation with a Kombucha Consortium. Nutrients 2018, 10, 1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vajrabhaya, L.-O.; Korsuwannawong, S. Cytotoxicity evaluation of a thai herb using tetrazolium (MTT) and sulforhodamine b (SRB) assays. J. Anal. Sci. Technol. 2018, 9, 15. [Google Scholar] [CrossRef] [Green Version]
- Andrews, J.H. Biological control in the phyllosphere. Annu. Rev. Phytopathol. 1992, 30, 603–635. [Google Scholar] [CrossRef] [PubMed]
- Elad, Y.; Kirshner, B. Survival in the phylloplane of an introduced biocontrol agent (Trichoderma harzianum) and populations of the plant pathogen Botrytis cinerea as modified by abiotic conditions. Phytoparasitica 1993, 21, 303. [Google Scholar] [CrossRef]
- Peteu, S.F.; Oancea, F.; Sicuia, O.A.; Constantinescu, F.; Dinu, S. Responsive polymers for crop protection. Polymers 2010, 2, 229–251. [Google Scholar] [CrossRef] [Green Version]
- Nix-Stohr, S.; Moshe, R.; Dighton, J. Effects of Propagule Density and Survival Strategies on Establishment and Growth: Further Investigations in the Phylloplane Fungal Model System. Microb. Ecol. 2008, 55, 38–44. [Google Scholar] [CrossRef]
- Li, Y.-Q.; Song, K.; Li, Y.-C.; Chen, J. Statistical culture-based strategies to enhance chlamydospore production by Trichoderma harzianum SH2303 in liquid fermentation. J. Zhejiang Univ. Sci. B 2016, 17, 619–627. [Google Scholar] [CrossRef] [Green Version]
- van der Burgh, A.M.; Joosten, M.H.A.J. Plant Immunity: Thinking Outside and Inside the Box. Trends Plant Sci. 2019, 24, 587–601. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Valdespino, C.A.; Casas-Flores, S.; Olmedo-Monfil, V. Trichoderma as a Model to Study Effector-Like Molecules. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef]
- de Torres Zabala, M.; Littlejohn, G.; Jayaraman, S.; Studholme, D.; Bailey, T.; Lawson, T.; Tillich, M.; Licht, D.; Bölter, B.; Delfino, L.; et al. Chloroplasts play a central role in plant defence and are targeted by pathogen effectors. Nat. Plants 2015, 1, 15074. [Google Scholar] [CrossRef]
- Stael, S.; Kmiecik, P.; Willems, P.; Van Der Kelen, K.; Coll, N.S.; Teige, M.; Van Breusegem, F. Plant innate immunity – sunny side up? Trends Plant Sci. 2015, 20, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Serrano, I.; Audran, C.; Rivas, S. Chloroplasts at work during plant innate immunity. J. Exp. Bot. 2016, 67, 3845–3854. [Google Scholar] [CrossRef]
- Peng, K.C.; Lin, C.C.; Liao, C.F.; Yu, H.C.; Lo, C.T.; Yang, H.H.; Lin, K.C. L-amino Acid Oxidase Secreted by Trichoderma Elicits Systemic Resistance by Transporting to Chloroplasts of Host Cells and Targeting Chlorophyll a/b Binding Proteins. Mol. Plant-Microbe Interact. 2019, 32, 111. [Google Scholar]
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants Application in Horticultural Crops under Abiotic Stress Conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef] [Green Version]
- Abreu, P.P.; Souza, M.M.; de Almeida, A.-A.F.; Santos, E.A.; Freitas, J.C.d.O.; Figueiredo, A.L. Photosynthetic responses of ornamental passion flower hybrids to varying light intensities. Acta Physiol. Plant. 2014, 36, 1993–2004. [Google Scholar] [CrossRef]
- Szymanska, R.; Slesak, I.; Orzechowska, A.; Kruk, J. Physiological and biochemical responses to high light and temperature stress in plants. Environ. Exp. Bot. 2017, 139, 165–177. [Google Scholar] [CrossRef]
- Chen, S.C.; Ren, J.J.; Zhao, H.J.; Wang, X.L.; Wang, T.H.; Jin, S.D.; Wang, Z.H.; Li, C.Y.; Liu, A.R.; Lin, X.M.; et al. Trichoderma harzianum Improves Defense Against Fusarium oxysporum by Regulating ROS and RNS Metabolism, Redox Balance, and Energy Flow in Cucumber Roots. Phytopathology 2019, 109, 972–982. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Téllez, V.I.; Cruz-Olmedo, A.K.; Plasencia, J.; Gavilanes-Ruíz, M.; Arce-Cervantes, O.; Hernández-León, S.; Saucedo-García, M. The Protective Effect of Trichoderma asperellum on Tomato Plants against Fusarium oxysporum and Botrytis cinerea Diseases Involves Inhibition of Reactive Oxygen Species Production. Int. J. Mol. Sci. 2019, 20, 2007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, J.; Liu, Z.; Li, Z.; Wang, Y.; Yang, K. Alleviation of the effects of saline-alkaline stress on maize seedlings by regulation of active oxygen metabolism by Trichoderma asperellum. PLoS ONE 2017, 12, e0179617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souza, P.U.; Lima, L.K.S.; Soares, T.L.; Jesus, O.N.d.; Coelho Filho, M.A.; Girardi, E.A. Biometric, physiological and anatomical responses of Passiflora spp. to controlled water deficit. Sci. Hortic. 2018, 229, 77–90. [Google Scholar] [CrossRef]
- Drake, P.L.; de Boer, H.J.; Schymanski, S.J.; Veneklaas, E.J. Two sides to every leaf: Water and CO2 transport in hypostomatous and amphistomatous leaves. New Phytol. 2019, 222, 1179–1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tosens, T.; Laanisto, L. Mesophyll conductance and accurate photosynthetic carbon gain calculations. J. Exp. Bot. 2018, 69, 5315–5318. [Google Scholar] [CrossRef]
- Romanatti, P.V.; Rocha, G.A.; Veroneze Júnior, V.; Santos Filho, P.R.; de Souza, T.C.; Pereira, F.J.; Polo, M. Limitation to photosynthesis in leaves of eggplant under UVB according to anatomical changes and alterations on the antioxidant system. Sci. Hortic. 2019, 249, 449–454. [Google Scholar] [CrossRef]
- Zou, M.; Yuan, L.; Zhu, S.; Liu, S.; Ge, J.; Wang, C. Effects of heat stress on photosynthetic characteristics and chloroplast ultrastructure of a heat-sensitive and heat-tolerant cultivar of wucai (Brassica campestris L.). Acta Physiol. Plant. 2016, 39, 30. [Google Scholar] [CrossRef]
- Latif, H.H.; Mohamed, H.I. Exogenous applications of moringa leaf extract effect on retrotransposon, ultrastructural and biochemical contents of common bean plants under environmental stresses. S. Afr. J. Bot. 2016, 106, 221–231. [Google Scholar] [CrossRef]
- Guo, L.; Chen, A.; He, N.; Yang, D.; Liu, M. Exogenous silicon alleviates cadmium toxicity in rice seedlings in relation to Cd distribution and ultrastructure changes. J. Soils Sediments 2018, 18, 1691–1700. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B.S. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, U.B.; Malviya, D.; Singh, S.; Kumar, M.; Sahu, P.K.; Singh, H.V.; Kumar, S.; Roy, M.; Imran, M.; Rai, J.P.; et al. Trichoderma harzianum- and Methyl Jasmonate-Induced Resistance to Bipolaris sorokiniana Through Enhanced Phenylpropanoid Activities in Bread Wheat (Triticum aestivum L.). Front. Microbiol. 2019, 10, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogt, T. Phenylpropanoid Biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrington, Y.; Guo, J.; Le, C.H.; Fillo, A.; Kwon, J.; Tran, L.T.; Ehlting, J. Evolution of a secondary metabolic pathway from primary metabolism: Shikimate and quinate biosynthesis in plants. Plant J. 2018, 95, 823–833. [Google Scholar] [CrossRef]
- de Oliveira, D.M.; Finger-Teixeira, A.; Rodrigues Mota, T.; Salvador, V.H.; Moreira-Vilar, F.C.; Correa Molinari, H.B.; Craig Mitchell, R.A.; Marchiosi, R.; Ferrarese-Filho, O.; Dantas dos Santos, W. Ferulic acid: A key component in grass lignocellulose recalcitrance to hydrolysis. Plant Biotechnol. J. 2015, 13, 1224–1232. [Google Scholar] [CrossRef]
- Shukla, N.; Awasthi, R.P.; Rawat, L.; Kumar, J. Seed biopriming with drought tolerant isolates of Trichoderma harzianum promote growth and drought tolerance in Triticum aestivum. Ann. Appl. Biol. 2015, 166, 171–182. [Google Scholar] [CrossRef]
- Czarnocka, W.; Karpiński, S. Friend or foe? Reactive oxygen species production, scavenging and signaling in plant response to environmental stresses. Free Radic. Biol. Med. 2018, 122, 4–20. [Google Scholar] [CrossRef]
- Ehonen, S.; Yarmolinsky, D.; Kollist, H.; Kangasjärvi, J. Reactive Oxygen Species, Photosynthesis, and Environment in the Regulation of Stomata. Antioxid. Redox Signal. 2018, 30, 1220–1237. [Google Scholar] [CrossRef] [PubMed]



| Variant | Chloroplast Number (n = 100) | Chloroplast Surface, µm2 (n = 40) |
|---|---|---|
| C—control (no treatment with plant biostimulants, treated only with water) | 7.9 ± 0.4b | 4.8 ± 0.8b |
| T1—foliar treatment with Trichoderma consortium suspension, 106 cfu/mL, equiv. to 1011 spores/ha | 8.4 ± 0.3b | 5.3 ± 0.6b |
| T2—foliar treatment with Trichoderma consortium suspension, 108 cfu/mL, equiv. to 1013 spores/ha | 12.8 ± 0.7a | 9.2 ± 1.1a |
| T3—foliar treatment with a reference product, consisting of plant extracts, essential oils and fatty acids in an oil/water emulsion, and extract of the seaweed Ascophyllum nodosum, equiv. to 3 L/ha | 8.9 ± 0.6b | 6.2 ± 0.9b |
| No. | Treatment | Marketable Yield, Dried Weight (Kg/15 Plants) | Total Polyphenols Harvested (g/15 Plants) | Total Flavonoids Harvested (g/15 Plants) |
|---|---|---|---|---|
| C | Control (untreated) | 0.90 ± 0.04b | 15.48 ± 2.06b | 11.16 ± 2.48b |
| T1 | Trichoderma 106 ufc/mL, equiv. 2 × 1011 spores/ha | 0.92 ± 0.06b | 16.37 ± 2.82b | 11.77 ± 2.84b |
| T2 | Trichoderma 108 ufc equiv. 2 × 1013 spores/ha | 1.15 ± 0.05a | 27.14 ± 4.73a | 21.39 ± 4.23a |
| T3 | Reference product, plant oil, algae, and plant extract | 1.01 ± 0.08ab | 18.48 ± 4,27ab | 14.24 ± 4.05b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Şesan, T.E.; Oancea, A.O.; Ştefan, L.M.; Mănoiu, V.S.; Ghiurea, M.; Răut, I.; Constantinescu-Aruxandei, D.; Toma, A.; Savin, S.; Bira, A.F.; et al. Effects of Foliar Treatment with a Trichoderma Plant Biostimulant Consortium on Passiflora caerulea L. Yield and Quality. Microorganisms 2020, 8, 123. https://doi.org/10.3390/microorganisms8010123
Şesan TE, Oancea AO, Ştefan LM, Mănoiu VS, Ghiurea M, Răut I, Constantinescu-Aruxandei D, Toma A, Savin S, Bira AF, et al. Effects of Foliar Treatment with a Trichoderma Plant Biostimulant Consortium on Passiflora caerulea L. Yield and Quality. Microorganisms. 2020; 8(1):123. https://doi.org/10.3390/microorganisms8010123
Chicago/Turabian StyleŞesan, Tatiana Eugenia, Anca Olguța Oancea, Laura Mihaela Ştefan, Vasile Sorin Mănoiu, Marius Ghiurea, Iuliana Răut, Diana Constantinescu-Aruxandei, Agnes Toma, Simona Savin, Adriana Florina Bira, and et al. 2020. "Effects of Foliar Treatment with a Trichoderma Plant Biostimulant Consortium on Passiflora caerulea L. Yield and Quality" Microorganisms 8, no. 1: 123. https://doi.org/10.3390/microorganisms8010123